Neutrons, one of the three fundamental particles that make up an atom, play a crucial role in the structure and behaviour of atomic nuclei. While neutrons are electrically neutral, they are not passive particles. They can interact with each other through the processes of attraction and repulsion, which are essential for understanding the behaviour of atomic nuclei.
The Nature of Neutrons
Neutrons are subatomic particles found in the nucleus of an atom, along with protons. Unlike protons, which carry a positive charge, neutrons have no charge, making them electrically neutral. This lack of charge allows neutrons to interact uniquely with other particles.
Attraction Between Neutrons
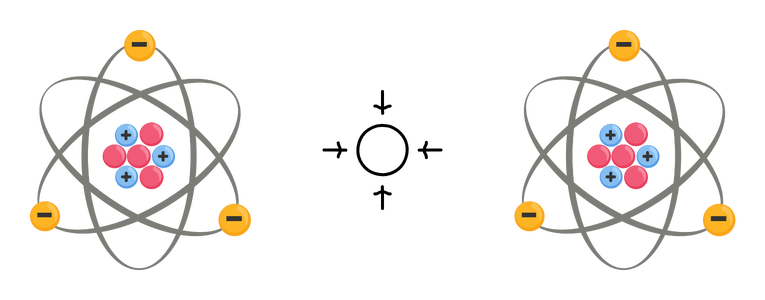
Attraction between neutrons occurs due to the strong nuclear force, also known as the strong interaction or vital force. This force holds the nucleus together, overcoming the electrostatic repulsion between positively charged protons. The strong force is one of the four fundamental forces in nature, along with gravity, electromagnetism, and the weak force.
Inside the nucleus, the vital force acts between all nucleons (protons and neutrons) and is much stronger than the electromagnetic force. This essential force overcomes the electrostatic repulsion between protons, allowing atomic nuclei to exist. However, the vital force also acts between neutrons, leading to attraction between them.
Repulsion Between Neutrons

While neutrons can attract each other, they can also experience repulsion under certain circumstances. This repulsion arises from the Pauli exclusion principle, which states that no two identical fermions (particles with half-integer spin, such as neutrons) can simultaneously occupy the same quantum state.
According to the Pauli exclusion principle, neutrons in a nucleus must occupy different quantum states, which means they cannot have the same energy, momentum, and spin. As a result, if two neutrons are forced into the same quantum state, they will experience repulsion due to their identical nature.
The Role of Neutron-Proton Interactions
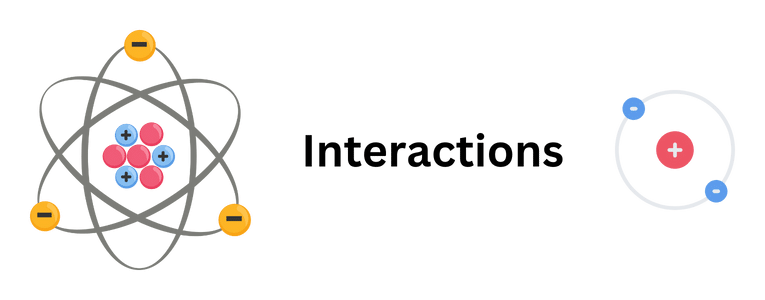
Neutrons not only interact with each other but also with protons. The interaction between neutrons and protons is crucial for determining the stability and properties of atomic nuclei. When neutrons and protons are close to each other, they can exchange particles known as mesons, which are carriers of the vital force.
This exchange of mesons between neutrons and protons leads to an attractive force between them, contributing to the overall stability of the nucleus. The balance between the attractive strong force and the repulsive electromagnetic force determines the size and shape of atomic nuclei.
The Impact on Nuclear Stability
The interplay between attraction and repulsion between neutrons significantly impacts the stability of atomic nuclei. The strength of a nucleus depends on the ratio of neutrons to protons, known as the neutron-proton ratio. Different isotopes of elements have different neutron-proton ratios, and this ratio affects the stability and behaviour of the nucleus.
If the neutron-proton ratio is too high or too low, the nucleus becomes unstable and can undergo radioactive decay. The nucleus may emit particles or radiation to achieve a more stable configuration in such cases. Understanding the processes of attraction and repulsion between neutrons is crucial for predicting and explaining the behaviour of radioactive isotopes.
Conclusion
Neutrons, as electrically neutral particles, interact with each other through the processes of attraction and repulsion. The strong nuclear force allows neutrons to attract each other, contributing to the stability of atomic nuclei. However, the Pauli exclusion principle leads to repulsion between neutrons when they occupy the same quantum state. The interplay between these forces determines the stability and behavior of atomic nuclei, ultimately shaping the world of nuclear physics.